Digital Transformation of Lab Testing Services for Precision Healthcare
Danner Laboratory was founded 30 years ago on the principles of delivering the best in accuracy, quality, and service. Our founder, Douglas E. Danner, CT(ASCP), has a passion for accuracy. Understanding how our work impacts patients has been the driving factor in maintaining excellence as a laboratory testing center. From the accuracy of our results, fast turnaround time, accessibility, and personalized customer service, we have built a reputation for being a trusted partner for laboratory testing and outsourcing.
Since its inception in 1991, Danner Laboratory established an outstanding track record of adopting advanced technology, including being the first laboratory in the Western USA to obtain ThinPrep Imager in 2003. As a CLIA-certified lab, Danner Laboratory has assembled a team of qualified and licensed cytotechnologists, pathologists, and laboratory scientists to work diligently by its established highest-quality systems to deliver clinical testing excellence.
Mission and Vision
Danner Laboratory aims to become the preeminent digital precision laboratory medicine research and clinical service provider on the West Coast and Canada, specializing in oncology and infectious disease diagnosis.
Our Mission is to provide accurate, timely, cost-effective, and valuable research and clinical information services. Our clients can count on us in clinical laboratory testing, so they can focus on taking care of their patients with the most well-informed healthcare decisions. Our vision is to empower health, save lives, and reveal better life through affordable, widely, and precisely accessible diagnostic tests.
Core Values
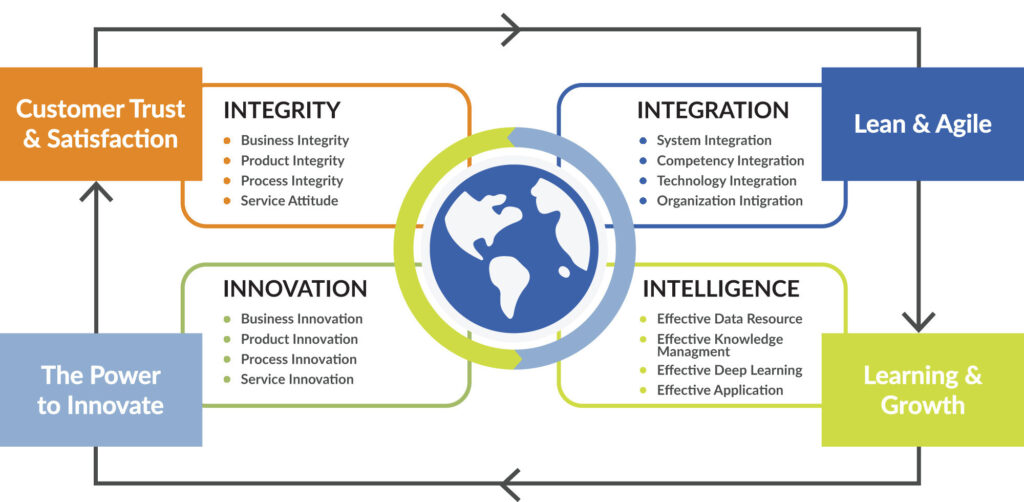
Behind every lab requisition and every sample being tested is an individual. This individual and their family can count on us to deliver laboratory service excellence, from accurate test results to timely reporting and personalized customer service. Our core values include customer trust and satisfaction, maintaining lean and agile infrastructure, a keen focus on innovation, and continued growth through effective data management, evaluation, and application.
The patient is at the forefront of everything we do. Our processes, decisions, and actions are driven by integrity. In addition to strictly complying with the laws and regulations governing our business, we are honest and forthright in our dealings with our customers and with each other.
As a company and as individual employees, we accept full responsibility for our performance and take accountability for the outcome of all that we do. We believe in teamwork and the limitless possibilities of collaborative energy. We achieve excellence by putting collective goals ahead of personal interests.
We are responsible corporate citizens in the community we serve. Our business decisions, including lab supply selection, waste management, water consumption, and energy usage, are made with the environment in mind.
Danner Labratory Business Principles
Danner Labratory Business Principles
A Legacy of Industry Innovation
Douglas E. Danner, CT(ASCP), founder of Danner Laboratory, graduated from the University of Oklahoma with degrees in Microbiology and Chemistry as well as graduating from the OU School of Cytotechnologists. He earned two U.S. Army fellowships at Memorial Sloan Kettering Hospital for Cancer and Allied Diseases in New York City where he studied under distinguished pathologists Myron Melamed, Steven Hajdu, and Leopold Koss. Doug also studied under Dr. Stanley F. Patten, who developed precise criteria for “Diagnostic Cytology” allowing more accurate and precise correlations between cytology and biopsy.
Under his leadership, Danner Laboratory was founded in San Diego, USA . The laboratory focused on test result accuracy, fast turnaround time and reliable service. Danner Laboratory quickly built a strong reputation for being a trusted partner of gynecology groups and clinics in the San Diego regions. When the Centers for Medicare & Medicaid Services (“CMS”) started regulating laboratory testing, Danner Laboratory gained its license under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”). The lab further organized and built on its business with a legal incorporation under the name of Danner Laboratory, Inc. in 2002, adoption of advanced ThinPrep technology in 2003, and addition of women health screening tests including Human papillomavirus (“HPV”), Chlamydia trachomatis/Neisseria gonorrhoeae (“GC/CT”), and Chlamydia trachomatis(“CT”).
In 2015, Danner Laboratory was acquired by General Biologicals Corporation (“GBC”), a diagnostic product manufacturer based in Taiwan. GBC added additional testing capabilities including molecular testing and immunoassays, and expanded the business operations to Canada with a new lab in Greater Vancouver, Canada (“Danner Laboratory, Vancouver” or “Danner Lab”). The new lab was provisionally accredited by the College of Physicians and Surgeons of BC under the Diagnostic Accreditation Program (“DAP”) in June 2022.


